electronic configuration for iodine|Iodine, electron configuration : Tagatay The total number of electrons in iodine is fifty-three. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in iodine in specific rules in different orbits and orbitals is called the electron configurationof . Tingnan ang higit pa Oglądaj darmowe seriale i filmy online bez limitu na Vider. Szeroki wybór tytułów, wysoka jakość i brak rejestracji. Twoje ulubione filmy i seriale za darmo, dostępne od zaraz! Witamy Witryna prosi o zgodę na wykorzystanie Twoich danych. Spersonalizowane reklamy i treści, pomiar reklam i treści, badanie odbiorców i ulepszanie usług .
PH0 · Iodine, electron configuration
PH1 · Iodine Electron Configuration: Everything You Need to Know
PH2 · Iodine Electron Configuration: Everything You Need to Know
PH3 · Iodine Electron Configuration (I) with Orbital Diagram
PH4 · Iodine (I)
PH5 · Iodine
PH6 · Electron Configuration Chart of All Elements (Full Chart)
PH7 · Electron Configuration
PH8 · Complete Electron Configuration for Iodine (I, I– ion)
PH9 · A step
PH10 · 7.3: Electron Configurations of Atoms
The story behind the making of Guardians of the Galaxy Vol. 3 is worthy of a film all its own. Originally meant to be an early entry in the Marvel Cinematic Universe’s Phase 4 – and reportedly planned to establish a larger space-set story for the upcoming phases – fate intervened in 2018, when former Walt Disney Studios chairman Alan Horn .
electronic configuration for iodine*******Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of iodine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. In the iodine ground-state electron configuration, the last electrons of the 5p orbital are located in the . Tingnan ang higit pa
The total number of electrons in iodine is fifty-three. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in iodine in specific rules in different orbits and orbitals is called the electron configurationof . Tingnan ang higit paelectronic configuration for iodineThe total number of electrons in iodine is fifty-three. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in iodine in specific rules in different orbits and orbitals is called the electron configurationof . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons . Tingnan ang higit pa
After arranging the electrons, it is seen that the last shell of the iodine atom has seven electrons. Therefore, the valence electronsof iodine are seven. The elements . Tingnan ang higit pa
Mar 23, 2023
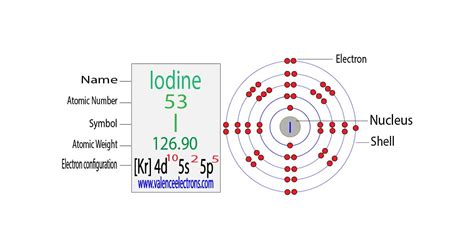
There are 53 electrons in iodine that occupy the respective orbitals as given below; 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁵. As 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ is the electronic configuration of the . In order to write the I electron configuration we first need to know the number of electrons for the I atom (there are 53 electrons). When we write the .Electron configuration for iodine. The history of Iodine. Periodic table history. Identifiers. List of unique identifiers for Iodine in various chemical registry databases. Iodine is a . Iodine (I) is located in the p-block of the periodic table. It belongs to period 5 and group 17. This article will discuss information on the electronic configuration of .
Iodine. Full electron configuration of iodine: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. tellurium ← iodine → xenon. Iodine, complete electron configuration. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be .a) Find the electron configuration of iodine [Kr] 5s 2 4d 10 5p 5. b) How many unpaired electrons does iodine have? To find the answer we refer to part a) and look at the valence electrons. We see that iodine has 5 . HALOGENS ELEMENT. Iodine was discovered by Bernard Courtois (FR) in 1811. The origin of the name comes from the Greek word iodes meaning violet. It is a shiny, .The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron .Iodine, electron configuration Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add .
I (Iodine) is an element with position number 53 in the periodic table. Located in the V period. Melting point: 113.5 ℃. Density: 4.94 g/cm 3 . Electronic configuration of the Iodine atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 5Electronic configuration of the Iodine atom in ascending . The chemical symbol of Iodine is I. The atom of Iodine has an atomic number of 53. The electronic configuration of Iodine is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁵. Iodine has seven valence electrons. The most common valency of Iodine is . In this video we will write the electron configuration for I- the Iodide ion. We’ll also look at why Iodine forms a 1- ion and how the electron configuration. Electron Configuration and Oxidation States of Iodine. Electron configuration of Iodine is [Kr] 4d10 5s2 5p5. Possible oxidation states are +1,5,7/-1. Electron Configuration. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical . The atomic number of iodine is 53, which means it has 53 electrons. Now it is possible to find the orbital notation of iodine very easily through electron configuration. That is, the orbital notation of iodine is 1s 2 2s 2 2p .electronic configuration for iodine Iodine, electron configuration Two of the lithium electrons can fit into the 1 s subshell, but the third electron must go into the second shell and the lower energy orbital, which is the 2 s orbital. Therefore, we write the electron configuration of a lithium atom as 1s22s1 (spoken as “one-ess-two two-ess-one”). The shell diagram for a lithium atom (Figure 2.7.1 2.7. 1 ).
Offering air-conditioned rooms in the Marikina district of Manila, Marion Hotel is 4.4 miles from Smart Araneta Coliseum. This condo hotel provides free private parking and daily room service. The condo hotel also offers facilities for disabled guests. At the condo hotel, all units are equipped with a desk, a flat-screen TV, a private bathroom .
electronic configuration for iodine|Iodine, electron configuration